Opposing Roles of IFN in Immunotherapy
Interferons Have Opposing Roles in Immune Homeostasis and Cancer
It is well-appreciated that type one interferon (IFN-I) and type two interferon (IFNG) are important immunostimulatory cytokines. Approaches to increase IFNs, such as radiation, can promote antigen presentation and stimulate dendritic cell function in order to facilitate T cell activation. However, besides these stimulatory effects, IFNs can also coordinate immune suppression. This inhibitory function of IFN is important in cases such as chronic pathogen infection. Here, IFN signaling can seemingly flip a switch to dampen persistent immune activation in order to protect the host from such dangers as immune-mediated pathology and autoimmunity. Given the widespread activation of anti-viral signaling in cancer, can tumors also exploit the inhibitory functions of IFN on the immune system?
IFN signaling can have favorable effects on the immune system but can also have inhibitory effects. Cancers can exploit this latter function to promote immune suppression.
Interferon Signaling in Cancer Cells Can Be Unfavorable In Contrast to Immune Cells
Cancer cells are notoriously adept at co-opting biological functions that favor cancer cell growth and survival. This includes harnessing the immunosuppressive functions of IFN to avoid immune-mediated attack and promote other aggressive features. As a consequence, IFN signaling and ISG expression by cancer cells has important implications for cancer immunotherapy. The effectiveness of immunotherapies like immune checkpoint blockade is influenced not only by the status of IFN signaling in immune cells, which is generally favorable, but also by the status of IFN signaling in cancer cells, which tumors have hijacked to exploit its immunosuppressive properties. These opposing effects of IFN signaling in cancer cells versus immune cells can be conceptualized by an ISG ratio that considers the level of ISG expression by both cell populations. In cancer patients, the ratio of ISGs in immune cells to ISGs in cancer cells can accordingly predict response to immune checkpoint blockade. But, does altering this ratio actually improve immune function and impact the outcome of immunotherapy?
IFN signaling, and hence ISG levels, in immune cells versus cancer cells have opposite effects on likelihood of clinical response to immune checkpoint blockade. Top shows expression of cancer-associated ISGs and immune-associated ISGs in melanoma patients colored by response to anti-PD1. Bottom shows relationship between the ISG ratio and response to immunotherapy.
Preventing IFN Signaling in Cancer Cells Increases IFN Signaling in Immune Cells
In mouse models of cancer, genetic engineering can be used to prevent IFN-I or IFNG signaling in cancer cells, resulting in a decrease in ISG expression. Interestingly, inhibiting ISG expression in cancer cells also unleashes a cascade of events that can broadly improve immune function. First, there is an increase in the abundance of CD8 T cells in the tumor. Moreover, these T cells are able to augment their generation of IFNG. This results in greater IFN signaling and ISG expression in various intratumoral immune cells, particularly innate immune cells like myeloid cells. These myeloid cells are then able to enhance production of stimulatory cytokines, which can positively affect other immune populations. Thus, the ISG ratio represents a feedback inhibition circuit whereby IFN signaling in immune cells limits its own activity by signaling through cancer cells. Blocking IFN signaling in cancer cells dramatically impacts the ISG ratio by changing both the numerator and the denominator, rendering resistant tumors now responsive to immunotherapy. However, given that cancers can utilize multiple ways to evade the immune system, how effective is blocking tumor IFN signaling as a strategy?
Single-cell RNA-sequencing analysis on immune cells in either wild type tumors or tumors with IFNG receptor deleted in cancer cells. Top plot is a density plot that shows the relative frequency of immune cells. Bottom plot shows expression levels of immune-associated ISGs.
Preventing IFN Signaling in Cancer Cells Enhances Killing by Both T Cells and Innate Immune Cells
To understand how effective blocking IFN signaling in cancer cells might be as a strategy, it is important to understand the immune mechanism by which response is improved. In the case of IFNG signaling, when cancer cells are prevented from signaling through the IFNG receptor both CD8 T cell killing and killing by innate immune cells are unleashed. For tumors with adequate neoantigen and MHC-I levels, this results in elimination predominantly by T cell-mediated cytotoxicity. For tumors with poor MHC-I and/or neoantigens, although CD8 T cells are unable to directly kill tumor cells, they can orchestrate tumor killing through innate immune cells. Here, the increased IFNG produced by T cells enhances IFN signaling in key immune populations that support NK and ILC1 maturation and killing function. Like IFNG signaling, blocking IFN-I signaling in tumor cells also improves immunotherapy efficacy; however, this utilizes a different immune mechanism. Thus, blocking IFN signaling in cancers cells might improve immunotherapy across many different scenarios of resistance.
Preventing cancer cell IFNG signaling enables CD8 T cells to kill tumors with adequate neoantigens and MHC-I expression. In tumors with poor neoantigens or MHC-I expression, the CD8 T cells cannot kill but the increased production of IFNG can promote tumor killing by NK/ILC1s.
Interferon Signaling Has Opposing Roles in Cancer Immunotherapy Response
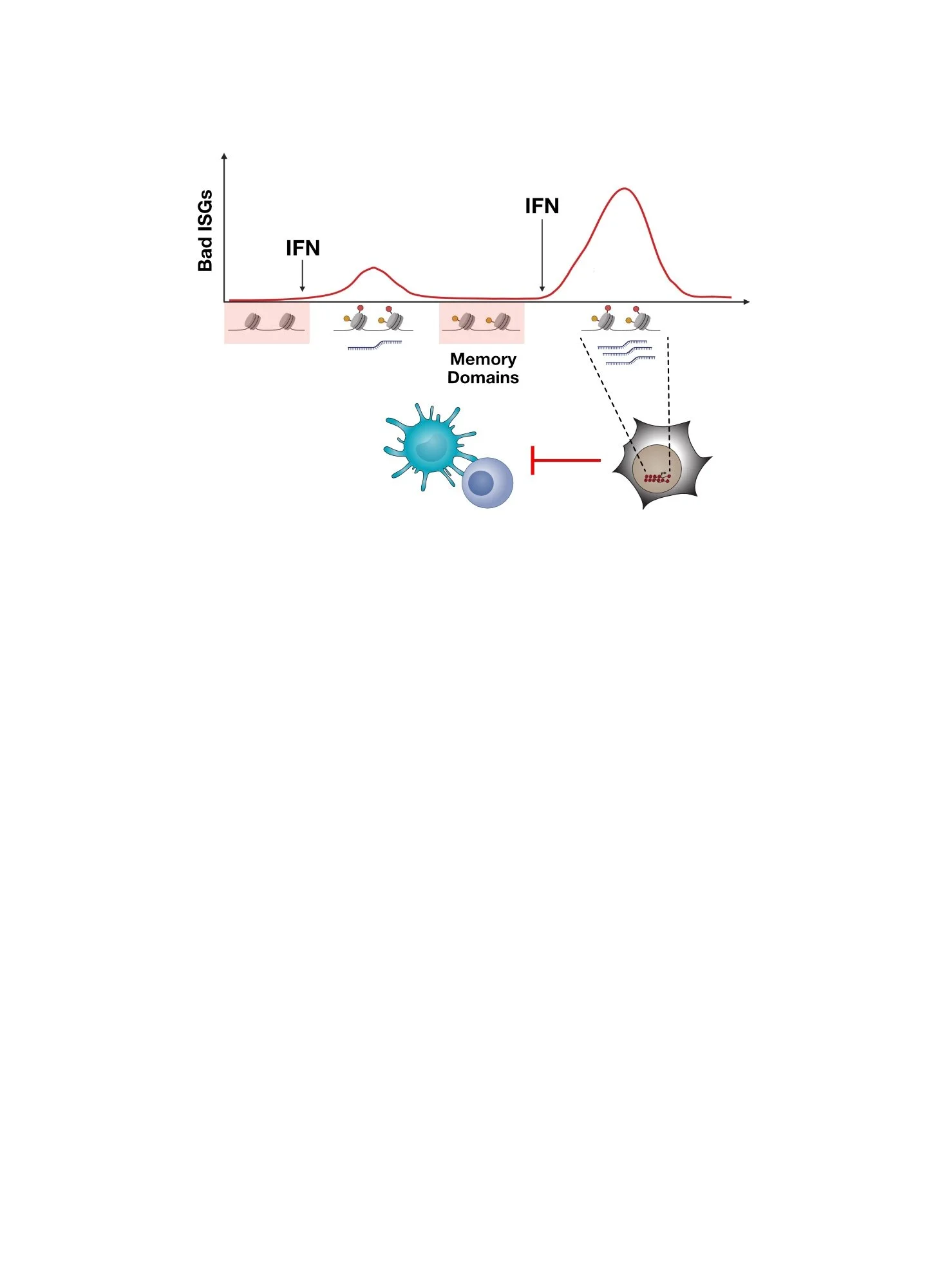
Together, our work suggests that IFN signaling has complex and even opposing roles in influencing cancer immunotherapy response. On the one hand, therapies such as radiation can be used to enhance immune checkpoint blockade by mimicking features of an acute virus infection. This promotes innate and adaptive immune function that can increase the likelihood of response. On the other hand, even combination and aggressive therapies often do not eradicate all disease. Remaining cancer cells may borrow cues from chronic virus infection. This includes exploiting properties of persistent IFN signaling to promote innate and adaptive immune suppression — a function of IFN signaling that typically is important in limiting immune-mediated pathology during non-resolving chronic pathogen infection.
Although the inhibitory feedback loop controlled by IFN signaling can be experimentally sabotaged in cancer models, whether the same can be achieved therapeutically is an on-going area of investigation. We are also investigating how specific ISGs expressed in cancer cells can control immune dysfunction and resistance, as described here, and why chronic IFN signaling makes cancer cells resistant, as described here.
Summary of the opposing functions of IFN signaling in cancer immunotherapy. Therapies such as radiation can enhance immune response by mimicking features of an acute virus infection. However, persistent disease may impose immune suppression by borrowing cues from a chronic virus infection.
Interested in reading more?
Visit our publications page for the full references to our work.
Benci et al, Cell 2016
Benci and Johnson et al, Cell 2019
Here Are Some of the People That Led The Studies and Made It Happen!
Joseph Benci, former grad student and post-doc
Lex Johnson, former grad student and post-doc
Darwin Ye, grad student
Jingya Qiu, grad student