Enhancing IFN and Antigenicity with CAR-T Cells
Diverting Interferon-Activating Stimuli Away from Cancer Cells To Promote Anti-Tumor Immunity
Since chronic interferon (IFN) signaling in cancer cells can promote immune dysfunction and immunotherapy resistance (discussed more here), this can present a problem for therapeutic strategies that seek to activate IFN signaling in the tumor microenvironment (TME) as a way to improve immunotherapy. In light of this, methods to preferentially activate IFN signaling in immune cells rather than cancer cells might enhance anti-tumor immunity while mitigating the risk of activating counter regulatory mechanisms driven by persistent IFN signaling in cancer cells. To explore the efficacy of this strategy, we needed:
An agonist that activates IFN signaling
A therapeutic method that can deliver this agonist toward immune cells and away from cancer cells.
For the agonist, we opted to use RN7SL1 (7SL), the endogenous RNA we previously discovered that can activate RNA pattern recognition receptors (PRRs) in the TME and enhance IFN signaling. But, what therapeutic method do we use to deliver it in the way we want?
Chronic IFN signaling in cancer cells can promote immunotherapy resistance. One strategy to improve the effectiveness of using agonists that activate IFN signaling in the TME is to deliver the agonist preferentially to immune cells. Shown here is the use of RN7SL1 (7SL), an endogenous RNA that activates RNA PRRs to increase IFN signaling.
Using CAR-T Cells To Preferentially Activate IFN Signaling in Immune Cells in the TME
Chimeric antigen receptor (CAR)-T cells are a breakthrough immunotherapy that starts with a patient’s own T cells and engineers it to kill tumors by giving it a CAR that can target specific proteins (CAR antigens) on the surface of cancer cells. After reintroducing these CAR-T cells to a patient, they typically do not rely on endogenous immune cells to kill cancer cells. This autonomous killing ability enables CAR-T cells to “go it alone” to attack cancer, which offers multiple advantages. However, there are also notable disadvantages. If cancer cells find a way to mask or lose the CAR antigen, the CAR is no longer activated, rendering cancer cells resistant to CAR-T cell killing. Moreover, since CAR-T cells are surrounded by endogenous immune cells, not purposefully engineering CAR-T cell to recruit the assistance of these endogenous immune populations seems to waste an opportunity to provide CAR-T cells with help, especially when CAR antigen loss occurs.
Given this untapped potential of CAR-T cells to activate endogenous immune cells, we teamed up with Dr. Carl June to test whether CAR-T cells could be used to deliver RN7SL1 preferentially to immune cells rather than cancer cells. If so, could this initiate productive IFN signaling in immune cell, avoid detrimental IFN signaling in cancer cells, and hence allow recruitment of endogenous immunity? If so, could this now provide CAR-T cells with a supporting cast to eradicate cancers, particularly those under threat of CAR antigen loss?
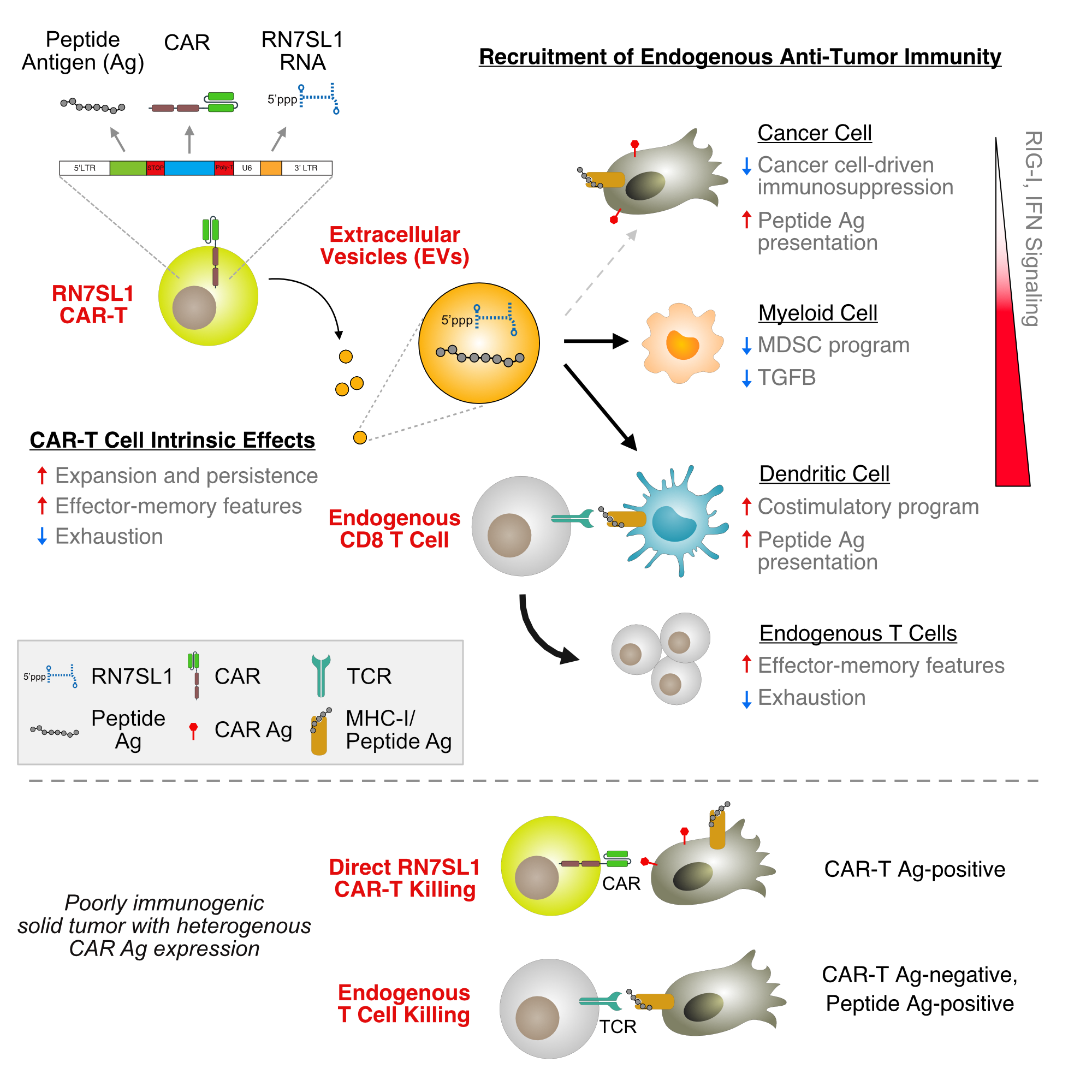
Design of CAR-T cells that deliver RN7SL1 using extracellular vesicles (EVs). CAR-T cell RNA in EVs is labelled with SytoRNA and transfer to cells in the tumor is followed by flow cytometry.
CAR-T Cells CAN Use Extracellular Vesicles to Deliver RN7SL1 Preferentially to Myeloid and Dendritic Cells
We engineered CAR-T cells to express RN7SL1 (RN7SL1-CAR-T cells) and discovered that CAR engagement results in increased production of extracellular vesicles (EVs) containing RN7SL1. This results in the preferential delivery of RN7SL1 to myeloid and dendritic cells (DCs) in the tumor rather than to cancer cells. As a consequence, several favorable changes occur in the innate immune cells. First, in contrast to what is observed after delivery of other types of PRR-activating RNAs, RN7SL1 prevents myeloid derived suppressor cell (MDSC) polarization. Second, RN7SL1 increases immunostimulatory features of DCs while decreasing negative immunoregulatory molecules. Thus, RN7SL1-CAR-T cells can productively activate important innate immune cells in the TME, which is the first step to activating T cells against the tumor.
RN7SL1 delivered by CAR-T cells promotes immunostimulatory features in myeloid and dendritic cells (DCs) in the tumor. RN7SL1 prevents MDSC polarization, increases plasmacytoid DC frequency, and increases expression of co-stimulatory genes (e.g., CD86) and decreases expression of inhibitory gene (e.g., PDL1) in DCs.
Endogenous ANTi-TUMOR T Cells Are Recruited by CAR-T Cells Delivering RN7SL1
Accompanying the productive activation of myeloid cells and DCs by RN7SL1-CAR-T cells is the expansion of endogenous CD8 T cells in the tumor. These CD8 T cells are reactive to tumor antigens and rather than becoming exhausted, or dysfunctional, these T cells develop effector-like properties. As a result, when combined with immune checkpoint blockade RN7SL1-CAR-T cells are significantly better than control CAR-T cells or CAR-T cells alone at eradicating tumors. Importantly, since endogenous tumor-reactive T cells can now help CAR-T cells kill cancer cells, even tumors with CAR antigen loss are effectively rejected. However, is this likely enough against most human cancers?
CAR-T cells that deliver RN7SL1 recruits endogenous tumor-reactive CD8 T cells with effector-memory features into the tumor. These RN7SL1-CAR-T cells can now reject tumors even when half of the cancer cells do not express the CAR antigen.
CAR-T Cells can Co-Deliver RN7SL1 and Peptide Antigen to Target Poorly Immunogenic Tumors
Although CAR-T cells can deploy RN7SL1 to recruit the endogenous immune system to help in rejecting tumors, endogenous CD8 T cells still need to recognize tumor antigens to be effective. Unfortunately, many human cancers lack good tumor antigens. To help overcome this problem, we engineered CAR-T cells to deliver RN7SL1 with an exogenous peptide antigen. This peptide antigen is also delivered by CAR-T cell EVs to both immune cells and cancer cells. Armed with both an RNA that enables innate immune cells to activate T cells and a peptide for T cell recognition, these CAR-T cells can now attack poorly immunogenic tumors. In fact, these CAR-T cells can even attack tumors with BOTH sparse neoantigens and CAR antigen loss — for many human solid tumors, we expect this challenging scenario will exist more often than not.
CAR-T cells can use EVs to co-deploy RN7SL1 and peptide antigen to endogenous immune cells and cancer cells to increase efficacy against poorly immunogenic tumors. Shown here is presentation of the peptide on the surface of cancer cells and immune cells. With both RN7SL1 and peptide antigen, these CAR-T cells are active even against KP lung cancer tumors, which have poor neoantigens AND is mixed 1:1 with CAR antigen negative cells.
RN7SL1 Expressed by CAR-T Cells Also Enhances Autonomous CAR-T Cell Function
Finally, although we engineered CAR-T cells to express RN7SL1 in order to recruit the endogenous immune system, we also examined whether autonomous CAR-T cell function was impacted. To our surprise, many intrinsic properties of CAR-T cells are also improved by expression of RN7SL1. This includes increased CAR-T cell persistence in the blood, greater infiltration into the tumor, and decreased CAR-T cell exhaustion. Consistent with this, in NSG mice that lack endogenous T cells, RN7SL1 also improves the ability of CAR-T cells to reject tumors.
In total, these CAR-T cells that co-deploy RN7SL1 and antigenic peptide have a collection of favorable properties that enable them to orchestrate a multi-pronged attack against anticipated barriers imposed by solid tumors. From our perspective, it all starts with recoding IFN signaling in the TME to promote a more immunostimulatory state. This allows RN7SL1-CAR-T cells to both kill independently of and recruit the endogenous T cell repertoire to help eradicate cancer. It can even provision tumors with new neoantigens. Despite this, the endogenous tumor-reactive T cell repertoire “is what it is”, which could be a remaining weakness. Is there a way to alter an endogenous T cell repertoire? Find out here.
Summary of how CAR-T cells that co-deliver RN7SL1 and peptide antigen can recruit endogenous anti-tumor immunity and enhance autonomous CAR-T cell function to improve efficacy, especially against tumors under threat of CAR antigen loss and/or that are poorly immunogenic.
Interested in Learning more?
Visit our publications page to find the full references for our work.
Johnson et al., Cell 2021
Here Are Some of the People That Led The Studies and Made It Happen!
Lex Johnson, former grad student and post-doc
Daniel Lee, former med student in year-out
Jaqueline Eacret, former post-doc